Introduction
Patient-Derived Xenografts (PDX) represent one of the most translationally relevant in vivo cancer models available to preclinical research. By directly implanting tumor fragments harvested from patients into immunodeficient mice, PDX models retain histological, genetic, and molecular fidelity to the original tumor. This allows researchers to capture tumor heterogeneity and therapeutic response patterns far more accurately than immortalized cell line–derived xenografts. PDX systems have become pivotal in bridging the gap between bench discoveries and clinical implementation.
Establishment of PDX Models
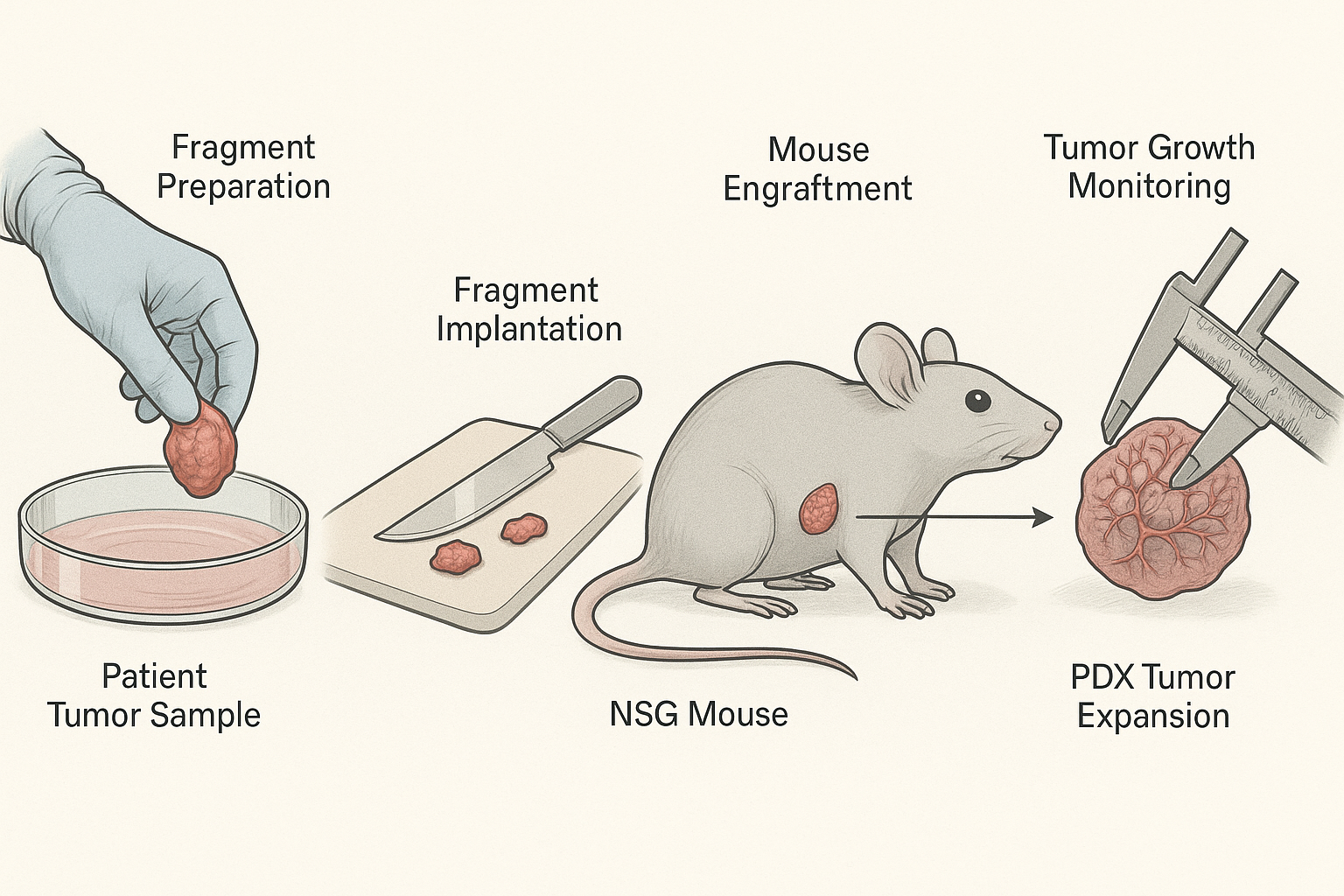
The process begins with the surgical acquisition of a primary or metastatic tumor specimen, usually under informed consent protocols. Tumor fragments (2–4 mm³) are immediately engrafted into recipient mice—commonly NSG or NOG strains—via subcutaneous or orthotopic implantation. Subcutaneous models provide ease of monitoring tumor growth, while orthotopic models replicate the native microenvironment of the tumor. Engraftment success rates depend on tumor type, aggressiveness, and stromal viability; hematological malignancies and poorly differentiated solid tumors often engraft more efficiently.
Engraftment Passaging and Expansion
Once established, tumors are passaged directly from mouse to mouse without in vitro culture, preserving architecture and clonal diversity. Early passages (P1–P3) retain the greatest fidelity to the patient sample, while higher passages risk genetic drift, stromal replacement, or selective clonal dominance. For large-scale studies, biobanking of early passage tumors is recommended to ensure reproducibility across projects.
Advantages of PDX Models
- Genomic and Transcriptomic Fidelity: They conserve mutations, gene expression signatures, and epigenetic states.
- Modeling Heterogeneity: Intratumoral diversity is preserved, allowing the study of resistant subpopulations.
- Clinical Correlation: Drug responses in PDX often mirror patient outcomes, supporting co-clinical trial designs.
- Biomarker Discovery: PDX enable biomarker validation linked to therapeutic sensitivity or resistance.
Limitations
Despite their advantages, PDX models are not without challenges. Murine stromal replacement occurs gradually, altering aspects of the tumor microenvironment. Establishment can be time-intensive, with engraftment requiring weeks to months. Additionally, conventional PDX models lack functional human immune systems, limiting their utility for immunotherapy studies unless paired with humanized host mice. Ethical and cost considerations also require careful oversight.
Workflow Summary
- Patient tumor specimen collection.
- Fragmentation and implantation into immunodeficient mice.
- Engraftment monitoring and tumor growth assessment.
- Serial passaging or cryopreservation for expansion.
- Experimental treatment and molecular profiling.
Future Outlook
The evolution of PDX is converging with advanced technologies such as CRISPR-mediated editing, humanized immune reconstitution, and single-cell multi-omics. These integrated approaches promise to transform PDX models into even more powerful tools for precision medicine, enabling mechanistic insights into resistance, biomarker discovery, and patient-specific therapy optimization.