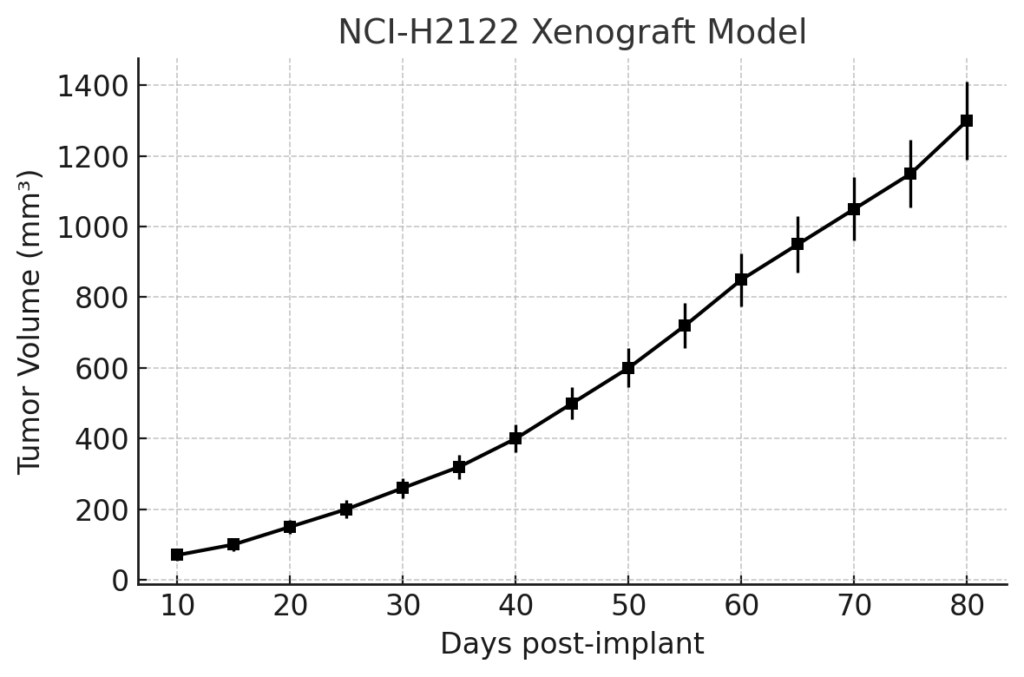
NCI-H2122 Xenograft Model Overview
The NCI-H2122 xenograft model is derived from a human lung adenocarcinoma and represents a KRAS-mutant, non-small cell lung cancer (NSCLC) subtype that is frequently associated with poor prognosis and resistance to EGFR-targeted therapies. The parental cell line was established from a lymph node metastasis and harbors the KRAS G12C mutation, one of the most clinically significant driver alterations in NSCLC. Due to its KRAS dependency and epithelial characteristics, the NCI-H2122 model provides a robust and biologically relevant system for evaluating KRAS-targeted therapies, immune-oncology agents, and combination regimens designed to overcome KRAS-mediated resistance mechanisms.
Request a Custom Quote for NCI‑H2122 Xenograft ModelBiological and Molecular Characteristics
NCI-H2122 cells exhibit epithelial morphology and are characterized by a canonical KRAS G12C mutation, resulting in constitutive activation of RAS signaling. The model lacks EGFR and ALK mutations, thereby serving as a clean background for studying KRAS-driven oncogenesis. The cell line also features loss-of-function mutations in STK11 (LKB1), a tumor suppressor involved in regulating cellular metabolism and immune microenvironmental interactions. NCI-H2122 is TP53 wild-type, and the expression of epithelial markers such as cytokeratin 7 and E-cadherin supports its epithelial tumor origin. These features provide a tractable system for dissecting the complex biology of KRAS-mutant lung adenocarcinomas.
| Characteristic | NCI-H2122 Cell Line Profile |
|---|---|
| Tumor Type | Lung adenocarcinoma (NSCLC) |
| KRAS Status | G12C mutation |
| EGFR/ALK Status | Wild-type |
| STK11 Status | Mutated (loss-of-function) |
| TP53 Status | Wild-type |
| Marker Expression | Cytokeratin 7⁺, E-cadherin⁺ |
In Vivo Model Development and Tumorigenicity
The NCI-H2122 xenograft model is developed by subcutaneous injection into immunodeficient mice, commonly NOD/SCID or athymic nude strains. Tumors typically develop within 10–14 days post-implantation and exhibit moderate growth kinetics, reaching volumes suitable for therapeutic intervention by 3–4 weeks. Due to its KRAS mutation status, the model exhibits partial resistance to EGFR inhibitors but is responsive to emerging KRAS G12C-targeted compounds, particularly those that engage the GDP-bound inactive form of KRAS. The model’s tumorigenicity is enhanced by its STK11-deficient background, which contributes to a distinct tumor microenvironment and supports investigations into immunotherapy resistance mechanisms.
Request a Custom Quote for NCI‑H2122 Xenograft ModelHistopathology and Immunohistochemical Profile
Histologically, NCI-H2122 xenografts produce moderately differentiated adenocarcinomas composed of polygonal tumor cells arranged in glandular and solid patterns. Tumors demonstrate cytoplasmic mucin content and show moderate nuclear pleomorphism. Immunohistochemistry confirms strong cytokeratin 7 and E-cadherin staining, reinforcing the epithelial phenotype. KRAS pathway activation is evident by the presence of phospho-ERK and phospho-MEK staining in untreated tumors, and these markers serve as pharmacodynamic readouts during treatment with KRAS inhibitors. Ki-67 indices typically range from 40–60%, supporting the model’s intermediate proliferative capacity.
Preclinical Applications and Drug Response
The NCI-H2122 xenograft model is a valuable tool in the preclinical assessment of KRAS G12C inhibitors, such as sotorasib and adagrasib, both as monotherapies and in rational drug combinations. The model’s STK11-deficient status makes it particularly relevant for studying resistance to immune checkpoint blockade and evaluating metabolic and epigenetic modulators that target STK11-related vulnerabilities. In addition to targeted therapy studies, NCI-H2122 is also utilized in biomarker discovery, tumor immune profiling, and mechanistic studies of KRAS-driven oncogenesis. The model supports both short-term efficacy trials and long-term survival studies, offering flexibility across a wide range of translational applications.
Request This Model
To incorporate the NCI-H2122 xenograft model into your preclinical oncology program focused on KRAS-driven lung cancer, contact us to access validated data, growth curves, and support for study design tailored to KRAS-targeted research.
Request a Custom Quote for NCI‑H2122 Xenograft Model