KU812 Xenograft Model Overview
The KU812 xenograft model is established from a human chronic myeloid leukemia (CML) cell line originally derived from the peripheral blood of a patient in the blast crisis phase. This cell line is widely used for modeling advanced-stage CML due to its expression of the Philadelphia chromosome t(9;22)(q34;q11), which encodes the constitutively active BCR-ABL1 fusion protein. KU812 cells also exhibit features of erythroid and basophilic differentiation, making them distinct from other CML lines. The xenograft model supports preclinical evaluation of BCR-ABL1–targeting tyrosine kinase inhibitors (TKIs), agents modulating erythroid or basophilic maturation, and combination strategies aimed at overcoming resistance in late-stage CML.
Request a Custom Quote for KU812 Xenograft ModelBiological and Molecular Characteristics
KU812 cells grow in suspension and exhibit a mix of basophilic and erythroid morphological features. They express erythroid-associated antigens such as glycophorin A, as well as myeloid and basophil markers including CD13, CD33, and FcεRI. The BCR-ABL1 fusion protein is the primary oncogenic driver, resulting in constitutive activation of multiple signaling cascades including JAK/STAT, PI3K/AKT, and RAS/RAF/MEK/ERK pathways. KU812 cells also exhibit increased expression of BCL2 and survivin, contributing to their anti-apoptotic phenotype. These molecular attributes position KU812 as a relevant tool for exploring BCR-ABL–mediated leukemogenesis, basophilic differentiation, and response to kinase inhibition.
| Characteristic | KU812 Cell Line Profile |
|---|---|
| Disease Origin | Chronic myeloid leukemia (blast crisis) |
| Key Genetic Alteration | BCR-ABL1 fusion (Philadelphia chromosome) |
| Immunophenotype | CD13⁺, CD33⁺, CD36⁺, Glycophorin A⁺, FcεRI⁺ |
| Differentiation Features | Erythroid-basophilic hybrid |
| Activated Pathways | BCR-ABL, PI3K/AKT, JAK/STAT, MAPK |
| Application | TKI efficacy, resistance studies, erythroid maturation |
In Vivo Model Development and Tumorigenicity
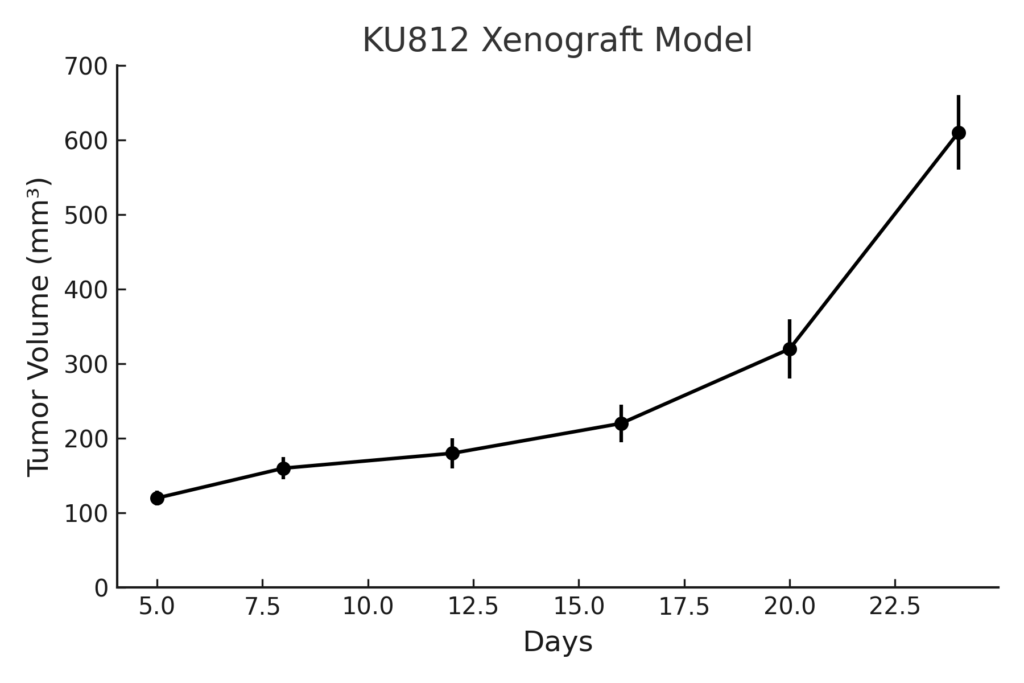
KU812 xenografts are generated through subcutaneous injection into immunodeficient mice such as NSG or NOD/SCID strains. Tumor formation occurs with moderate latency, and tumors typically reach 600–900 mm³ in volume within 4–6 weeks. Engraftment efficiency is high, and tumor progression is consistent across cohorts, making the model suitable for controlled therapeutic intervention studies. Intravenous injection can also be used to establish disseminated leukemia models, although these are less commonly applied due to limited infiltration efficiency. The subcutaneous model is preferred for monitoring tumor burden, evaluating pharmacodynamics, and conducting dose-response assessments.
Request a Custom Quote for KU812 Xenograft ModelHistopathology and Immunohistochemical Profile
Histologically, KU812-derived tumors consist of medium to large leukemic cells with basophilic cytoplasm, eccentrically placed nuclei, and frequent mitoses. H&E staining reveals a relatively homogenous tumor mass with minimal stromal support. Immunohistochemical analysis demonstrates strong membrane and cytoplasmic staining for glycophorin A and CD13, confirming erythroid and myeloid lineage traits. Ki-67 proliferation indices are typically elevated, correlating with rapid growth dynamics. Expression of phosphorylated STAT5 and ERK is evident in untreated tumors and can be used as pharmacodynamic markers in studies evaluating BCR-ABL or MAPK pathway inhibition.
Preclinical Applications and Drug Response
The KU812 xenograft model is highly responsive to first- and second-generation TKIs such as imatinib, dasatinib, and nilotinib, providing a validated platform for assessing kinase-targeted therapies. It is also utilized to evaluate agents that target downstream signaling cascades, including PI3K and STAT5 inhibitors. Due to its dual lineage phenotype, the model supports studies on erythroid differentiation and basophil-mediated inflammation, particularly in CML blast phase. In addition, the model facilitates investigation of resistance mechanisms related to BCR-ABL1 mutations, apoptotic pathway dysregulation, and survival signaling under selective drug pressure.
Request This Model
To incorporate the KU812 xenograft model into your chronic myeloid leukemia research or drug development studies, contact our scientific team for access to detailed model data, validated implantation protocols, and custom in vivo study support tailored to your research objectives.
Request a Custom Quote for KU812 Xenograft Model