EBC-1 Xenograft Model Overview
The EBC-1 xenograft model is derived from a human squamous cell carcinoma of the lung and serves as a highly relevant in vivo platform for studying non-small cell lung cancer (NSCLC) driven by MET amplification. Originating from a Japanese male patient, the EBC-1 cell line displays epithelial morphology and harbors high-level amplification of the MET proto-oncogene, resulting in constitutive activation of MET-dependent signaling pathways. When implanted subcutaneously into immunodeficient mice, EBC-1 cells produce fast-growing, consistent tumors, making the model particularly suited for evaluating MET-targeted therapeutics, resistance mechanisms, and rational drug combinations. Due to its defined oncogenic dependency, the EBC-1 xenograft model is widely used in translational research aimed at overcoming resistance to tyrosine kinase inhibitors and identifying novel vulnerabilities in MET-amplified NSCLC.
Request a Custom Quote for EBC‑1 Xenograft ModelBiological and Molecular Characteristics
The defining feature of the EBC-1 cell line is its strong MET gene amplification, which drives persistent activation of downstream PI3K/AKT and MAPK signaling cascades. The cell line lacks activating mutations in EGFR, KRAS, and ALK, making it an ideal system for selectively studying MET-targeted therapeutic agents. EBC-1 cells exhibit strong epithelial features, including cytokeratin 5/6 and E-cadherin expression, and moderate levels of PD-L1, providing insight into both oncogenic signaling and immune evasion. The MET dependency in EBC-1 also allows for detailed exploration of resistance mechanisms that arise through bypass pathway activation or secondary mutations.
| Characteristic | Description |
|---|---|
| Tissue Origin | Human lung squamous cell carcinoma |
| Dominant Alteration | MET amplification |
| Other Mutation Status | EGFR, KRAS, ALK wild-type |
| Cell Morphology | Epithelial, adherent |
| Immunomarkers | CK5/6+, E-cadherin+, PD-L1 (moderate) |
| Active Pathways | MET–PI3K/AKT, MET–MAPK |
In Vivo Model Development and Tumorigenicity
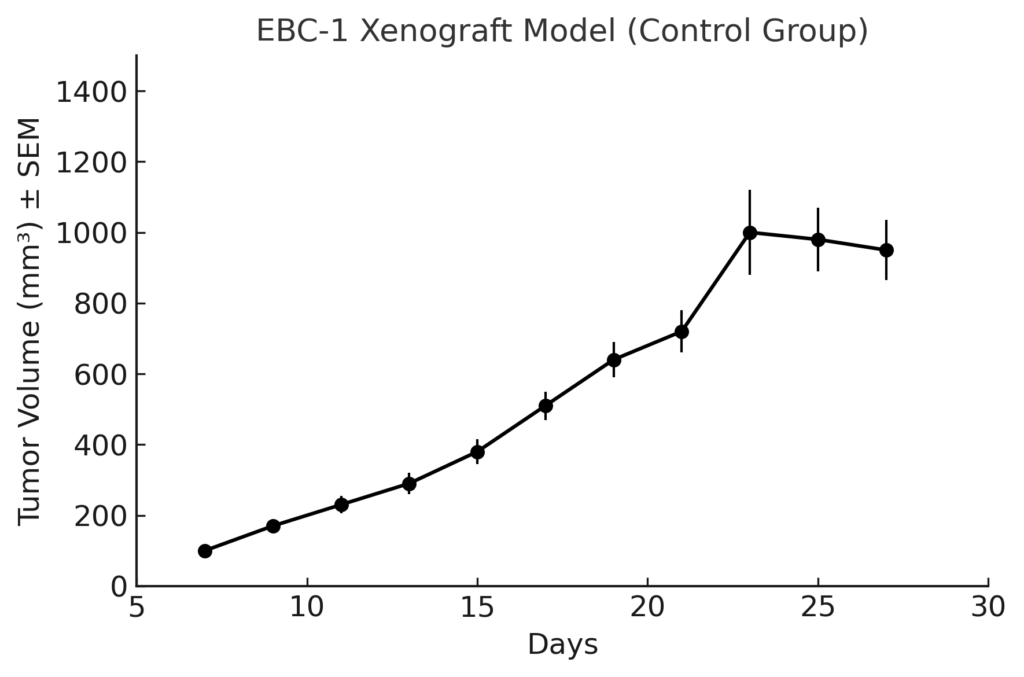
The EBC-1 xenograft model is established through subcutaneous injection of tumor cells into immunodeficient mouse strains such as NOD/SCID or athymic nude mice. Tumors typically develop within 7–10 days and grow rapidly to volumes of 300–500 mm³ over 3–5 weeks, enabling time-efficient therapeutic studies. Tumor take rates are consistently high, and growth kinetics are linear, providing a stable foundation for treatment efficacy evaluations. The model supports pharmacokinetic/pharmacodynamic studies, dose titration experiments, and tumor regression assessments under MET inhibitor treatment. Its reliability and specificity make it a favored choice for testing selective MET inhibitors, combination therapies targeting parallel pathways, and novel drug delivery systems.
Request a Custom Quote for EBC‑1 Xenograft ModelHistopathology and Immunohistochemical Profile
Histological examination of EBC-1 xenografts reveals poorly to moderately differentiated squamous carcinoma characterized by nests and sheets of polygonal tumor cells with intercellular bridges and keratinization in some regions. Hematoxylin and eosin staining confirms high nuclear-to-cytoplasmic ratios, vesicular chromatin, and frequent mitoses. Immunohistochemical analysis demonstrates strong cytoplasmic and membranous staining for MET, as well as positivity for squamous markers such as cytokeratin 5/6 and p63. E-cadherin is robustly expressed, affirming epithelial lineage. Ki-67 staining typically shows a proliferation index exceeding 60%, reflecting the model’s high mitotic activity. PD-L1 staining is moderate and heterogeneous, supporting its utility in immune combination therapy studies.
Preclinical Applications and Drug Response
The EBC-1 xenograft model is a cornerstone of MET-targeted drug development programs. It exhibits high sensitivity to selective MET inhibitors such as crizotinib, tepotinib, and capmatinib, and has been used to evaluate dose–response relationships and mechanisms of acquired resistance. Combination therapy studies involving EGFR inhibitors, PI3K/AKT inhibitors, and immune checkpoint blockade have shown enhanced activity in EBC-1 tumors, particularly under conditions of adaptive resistance or pathway redundancy. The model is also suitable for evaluating antibody–drug conjugates, bispecific antibodies targeting MET, and nanoparticle-based delivery systems. Its defined dependency on MET signaling makes it a powerful tool for both mechanistic interrogation and therapeutic validation.
Request This Model
To request the EBC-1 xenograft model for your preclinical studies, please use the form below. A customized quote and additional model specifications will be provided upon inquiry.
Request a Custom Quote for EBC‑1 Xenograft Model